Medical Water Purification Systems
Discover the world leader in dialysis water systems for the medical industry.
Commercial & Industrial Water Filtration Systems
Mar Cor is an industry leader in water filtration systems for commercial and industrial applications.

Services for Dialysis Facilities, Industrial Labs, and More!
Mar Cor offers a variety of service options to take the worry and expense out of water system maintenance.

We Create and Service The Best Water Filtration and Purification Systems In The World
Hospitals, dialysis clinics, industrial labs and commercial industries continue to choose Mar Cor for safe, reliable water purification systems, filtration products and professional equipment training.
To learn how we can help your organization increase production and serve patients with quality purified water solutions, contact us today to speak with a specialist!
News & Events
Find out the latest information regarding Mar Cor. Browse company news, industry updates, and more.

Featured Product
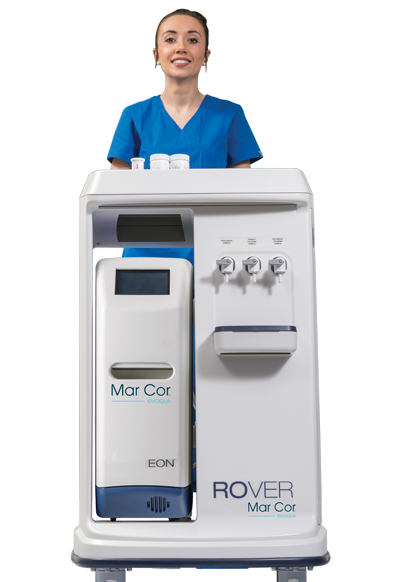
EON
Improve your dialysis water treatment experience with the EON portable RO system. This advanced solution quickly and efficiently produces and maintains safe, high-quality medical grade water. For our newest, most advanced RO water system, we combined reliable methodology with new technology to deliver a portable solution that medical teams can confidently and easily operate for single-patient hemodialysis treatments.







